Sterilization Services
- Full Sterilization Management
- EO, Gamma & Steam Sterilization
- ISO 17665 Certified
COMPLETE MEDICAL DEVICE STERILIZATION SERVICES
PRO-TECH offers full contract sterilization services, including validation and cycle development. We employ a team of microbiologists specializing in medical device sterilization. Furthermore, we have both in-house and managed third-party laboratory support.

COMMITMENT TO QUALITY
From cycle development to protocol to sample preparation to the final report, our customers enjoy a complete sterilization solution. We also provide ongoing regulatory support to ensure continued compliance. Our Quality Assurance group performs on-site audits of all our sterilization contractors at least annually and keeps scorecards of overall performance.
To meet your compliance needs, all cycles and sterility data are reviewed and approved by an on-staff microbiologist. Data and certificates are made available to customers 24/7 via our customer portal.

EXPERIENCE
Our customers benefit from our 40 years of experience in the industry. With sterilization, it’s important not only to be compliant but also to be efficient and time-sensitive. We design medical device sterilization programs to meet your specific product needs, and to ensure that they are cost effective. In most cases, we can even utilize our foundation of existing work to speed you to market. So, why start over? Why pay for half empty sterilization chambers? Why use a contractor with less sterilization experience than you have? Because, with PRO-TECH you don’t have to.
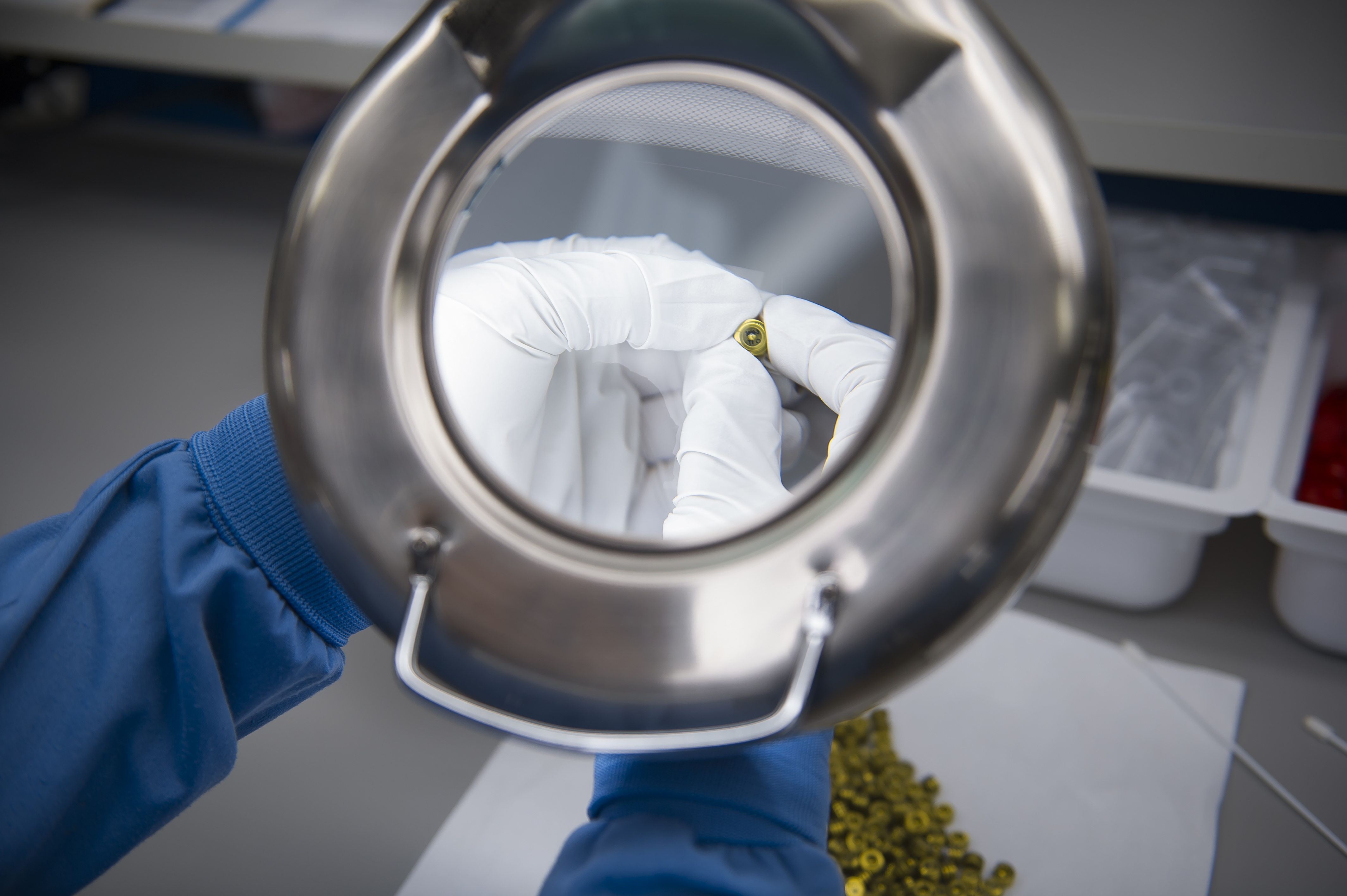
RANGE OF OPTIONS
We currently sterilize both implantable and non-implantable medical devices by ethylene oxide, gamma radiation, and steam.

ETHYLENE OXIDE
Ethylene Oxide (EO or ETO) is among the most common sterilization methods in the medical device industry. At PRO-TECH, we use it all the time. In fact, we ran nearly 2022, we ran nearly 1,900 pallets of EO sterilized product last year, in almost 190 cycles.
PRO-TECH runs mixed loads with products adopted into our iPCD-based product family, allowing our customers to pay a fraction of the sterilization cost. Adoption studies are performed in accordance with recognized standards such as ISO 11135 and AAMI TIR 28. You can leverage existing data supplemented with laboratory testing specific to your product. Common tests include: bioburden, LAL, comparative resistance, and EO residual studies
per ISO 10993-7.

STEAM STERILIZATION
PRO-TECH is an ISO 17665 registered contract steam sterilizer. We have both 6 and 56 cubic foot chambers located at our Santa Fe Springs, CA location. Contract services are available, whether PRO-TECH packages your device or not.
All our chambers are clean steam, GMP, and are capable of Air-Over-Pressure (AOP) to accommodate the needs of liquid products. These cycles are typically used for the terminal sterilization of liquid filled containers, tubes, vials, and syringes.
Our 6-cubic foot chamber is equipped with Steam-Air-Mix (SAM) capabilities for high pressure during exposure and cool down.
RADIATION
PRO-TECH sterilizes medical devices with both gamma and electron beam (E-Beam). We use local sterilization contractors throughout the country to save shipping costs and mitigate the risk of product damage during transit.
Our sterilization experts will guide you through the sterilization process of establishing your product family, executing the performance qualification of your dose, and provide ongoing dose audit support.
For more information on our sterilization capabilities, please
Sterilization Services and Capabilities
Pro-Tech Design offers full sterilization management services to our medical device customers
- ISO 17665 Certified Steam Sterilizer
- Strong Relationships with 3rd Party Sterilizers
- EO, Gamma, E-Beam, and Steam sterilization
- Validation and Cycle Development
- Experience matching packaging to sterilization method
- Mixed pallet loads minimize cost & time
Frequently Asked Questions
What is sterilization, and what are its benefits?
Sterilization is a critical process that occurs after the medical device has been assembled and placed in its final packaging. At this point, the device and its packaging may be contaminated with microorganisms, which can compromise the sterility of the device and potentially harm the patient. That’s why sterilization methods, such as ethylene oxide, gamma radiation, and steam, are used to eliminate any potential microbial contamination and ensure the safety and effectiveness of the device.
Sterilization comes with some of the following benefits:
- Ensuring safety: The primary benefit of sterilization is to ensure the safety of medical devices by eliminating any potential microbial contamination. Medical devices that are not properly sterilized can cause infections or other harmful complications for patients.
- Meeting regulatory requirements: Sterilization is a critical step in complying with regulatory requirements for medical devices. Regulatory bodies such as the FDA require that medical devices are sterilized before they can be sold or distributed to patients.
- Improving product quality: Sterilization can also help improve the quality of medical devices by ensuring that they are free from contaminants that can compromise their effectiveness or performance.
- Enhancing shelf life: Sterilization can extend the shelf life of medical devices by preventing the growth of microorganisms that can cause degradation or spoilage.
- Customization: Sterilization can be customized to meet the specific needs of different medical devices and their packaging, ensuring that the sterilization procedure is optimized for the product in question.
What are the packaging requirements for sterilization?
Packaging requirements for sterilization depend on the specific method of sterilization being used and the type of medical device being sterilized. However, there are some general packaging requirements that apply to most sterilization methods.
First, the packaging must be compatible with the sterilization method being used. For example, ethylene oxide sterilization requires gas-permeable packaging materials, while radiation sterilization may require different types of packaging materials.
Second, the packaging must be designed to protect the medical device from damage during sterilization and transportation. This may involve using cushioning materials or designing the packaging to withstand certain levels of pressure or vibration.
Third, the packaging must be designed to maintain the sterility of the device after sterilization. This may involve using sterile barriers or double-wrapping to prevent contamination during storage and transportation.
Fourth, the packaging must be clearly labeled with all necessary information, including the sterilization method used, the date of sterilization, and any relevant regulatory or compliance information.
At PRO-TECH, our team of experts works closely with clients to design customized packaging solutions that meet the specific requirements of their medical devices and the sterilization method being used. Our goal is to ensure that the packaging is optimized for both the sterilization process and the transportation and storage of the medical device while maintaining sterility and complying with regulatory requirements.
What is the difference between in-house and 3rd party sterilization?
We offer both in-house and third-party sterilization services, depending on the specific needs of our clients.
We are an ISO 17665 registered steam sterilizer and provide in-house steam sterilization services at our facility in Santa Fe Springs, CA. This helps us reduce leads times, control costs, and enhance flexibility.
We also work with 3rd party sterilizers who are experts in EO and Gamma Sterilization. FDA and EPA regulators have specific requirements that guide the health and safety of these sterilization techniques. As a result, we recommend working with experts in these forms of sterilization. We have strong relationships with EO and Gamma sterilizers and we are able to bundle different customer’s devices into single pallets. This reduces costs and timing for each customer and helps get them access to sterilizers, which can be difficult.
What are the benefits of working with PRO-TECH?
Choosing the right partner for medical device sterilization is crucial to ensure that products are effectively sterilized, compliant with regulatory requirements, and delivered to the market in a timely and cost-effective manner. PRO-TECH offers a range of contract sterilization services, and working with PRO-TECH offers several benefits, including:
- High-quality, cost-effective processing: As we already mentioned, our team of experts works closely with clients to design customized sterilization programs that meet their specific needs while ensuring compliance, efficiency, and cost-effectiveness. Our expertise in medical device sterilization, combined with our state-of-the-art facilities and equipment, ensures that your products are effectively sterilized while minimizing costs.
- Established network of sterilization facilities: We have a network of sterilization facilities that we work with, which enables us to provide fast turnaround times and flexible scheduling options for our clients. This allows you to get your products to market quickly and efficiently.
- Multidisciplinary technical staff and on-site quality assurance personnel: Our team of experts includes multidisciplinary technical staff and on-site quality assurance personnel, who are dedicated to ensuring the highest levels of quality and compliance in all aspects of the sterilization process. Our experts are there to assist throughout the entire process, from cycle development to protocol to sample preparation to the final report, and we provide ongoing regulatory support to ensure continued compliance.
- Detailed certification of your medical device sterilization process: We provide detailed certification of your medical device sterilization process, including data and certificates that are made available to customers 24/7 via our customer portal. Our Quality Assurance group performs audits of all our sterilization contractors at least annually and keeps scorecards of overall performance.
What is EtO or EO?
EtO and EO both stand for Ethylene Oxide, which is a colorless and flammable gas that is commonly used as a sterilization agent for medical devices. Ethylene oxide is a highly effective sterilant that is capable of penetrating the packaging and materials of medical devices to kill microorganisms such as bacteria, viruses, and fungi.
To learn more about EO sterilization and how it’s used in medical device packaging, visit this page.
What is the EO Cycle validation?
Ethylene oxide (EO) cycle validation is a critical step in the sterilization process for medical devices using ethylene oxide gas. EO cycle validation is the process of verifying that the sterilization cycle is capable of effectively sterilizing medical devices and achieving the desired level of sterility assurance.
The EO cycle testing process typically involves the following steps:
- Preparing a representative sample of the medical device to be sterilized.
- Exposing the sample to the sterilization cycle, which includes the specified conditions for temperature, humidity, and EO gas concentration.
- Monitoring the cycle using calibrated sensors and equipment to ensure that the specified conditions are maintained throughout the cycle.
- Performing biological and chemical tests on the sterilized sample to confirm the efficacy of the sterilization process.
- Documenting the results of the validation process, including the cycle parameters, test results, and any deviations or corrective actions taken.
EO cycle validation is a critical step in ensuring the safety and effectiveness of medical devices that are sterilized using ethylene oxide gas. It is also required by regulatory agencies such as the FDA to demonstrate compliance with regulatory requirements.
What are the different sterilization methods for medical devices?
Medical devices require effective sterilization to ensure that they are free from harmful microorganisms before use. There are several methods for sterilizing medical devices, such as moist heat (steam), dry heat, radiation, ethylene oxide gas, and vaporized hydrogen peroxide. Additionally, other sterilization methods such as chlorine dioxide gas, vaporized peracetic acid, and nitrogen dioxide may also be used.
- Moist heat sterilization, such as steam sterilization, is a common method for sterilizing medical devices that can withstand high temperatures and moisture. This method involves exposing the medical device to high-pressure steam, which kills microorganisms on the device.
- Dry heat sterilization, on the other hand, uses high temperatures to kill microorganisms on medical devices. This method is suitable for devices that can withstand high temperatures but may not be suitable for all medical devices.
- Radiation sterilization uses high-energy photons or electrons to kill microorganisms on medical devices. This method is suitable for a wide range of medical devices, but it may cause damage to certain materials, such as plastics.
- Ethylene oxide gas is another common method for sterilizing medical devices that can penetrate the packaging and materials of the device to kill microorganisms. This method is effective for a wide range of medical devices but requires careful handling due to its potential hazards.
- Vaporized hydrogen peroxide is a newer method for sterilizing medical devices that use gas to kill microorganisms. This method is effective for a wide range of medical devices, including those that cannot withstand high temperatures or moisture.
What are the benefits of terminal sterilization?
Terminal sterilization is a process of sterilizing medical devices at the end of the manufacturing process, typically just prior to use. There are several benefits of terminal sterilization, including:
- Increased safety: Terminal sterilization ensures that medical devices are free of viable microorganisms that could cause infection or harm to patients.
- Greater flexibility: Terminal sterilization allows for greater flexibility in the manufacturing process, as devices can be sterilized in bulk after production, rather than requiring aseptic processing throughout the manufacturing process.
- Cost-effectiveness: Terminal sterilization can be a cost-effective approach to ensuring the safety of medical devices, as it allows for bulk sterilization of devices and reduces the need for costly aseptic processing.
- Simplified regulatory compliance: Terminal sterilization is a well-established and widely accepted approach to sterilizing medical devices, making it easier to get regulatory approvals for device safety.
- Extended shelf-life: Terminal sterilization can extend the shelf life of medical devices by eliminating microbial growth and contamination that can occur during storage and transportation.
