The global medical device supply chain has never been more complex, and never more vital to get right.
For OEMs, contract packagers, and operations teams navigating a landscape shaped by shifting tariffs, reshoring pressures, and heightened regulatory scrutiny, one thing is clear: resilience isn’t a nice-to-have; it’s a survival skill.
With more than 45 years in the industry, PRO-TECH has seen supply chain landscapes shift under economic, political, and regulatory pressure. We’ve built a model that not only endures these changes but helps our clients thrive in them. We are also family-owned and operated, which gives us the flexibility to adapt quickly without having to work through layers of corporate decision-makers or remote private equity owners. Here’s how medical device manufacturers can do the same.
Why Medical Device Supply Chains Are Under Pressure in 2025
A decade ago, sourcing decisions were often dictated by price alone. Those days are gone. In 2025, manufacturers face a far more complex equation:
- Tariff fluctuations between the U.S. and major overseas manufacturing hubs
- Reshoring and nearshoring momentum driving operations closer to home
- Geopolitical instability and unpredictable logistics timelines
- Stricter compliance demands from the FDA, ISO 13485, and UDI traceability standards.
In today’s environment, how and where you produce matters as much as what you produce.
Reshoring and Nearshoring Impact
Many OEMs are rethinking global sourcing, moving away from dependency on far-flung vendors to reduce risk and increase responsiveness. Whether through full reshoring or selective nearshoring to North American partners, the advantages are significant:
- Shorter delivery timelines that keep projects on schedule
- Closer regulatory alignment with U.S. compliance expectations
- Clearer communication and faster resolution of issues
- Stronger intellectual property protection.
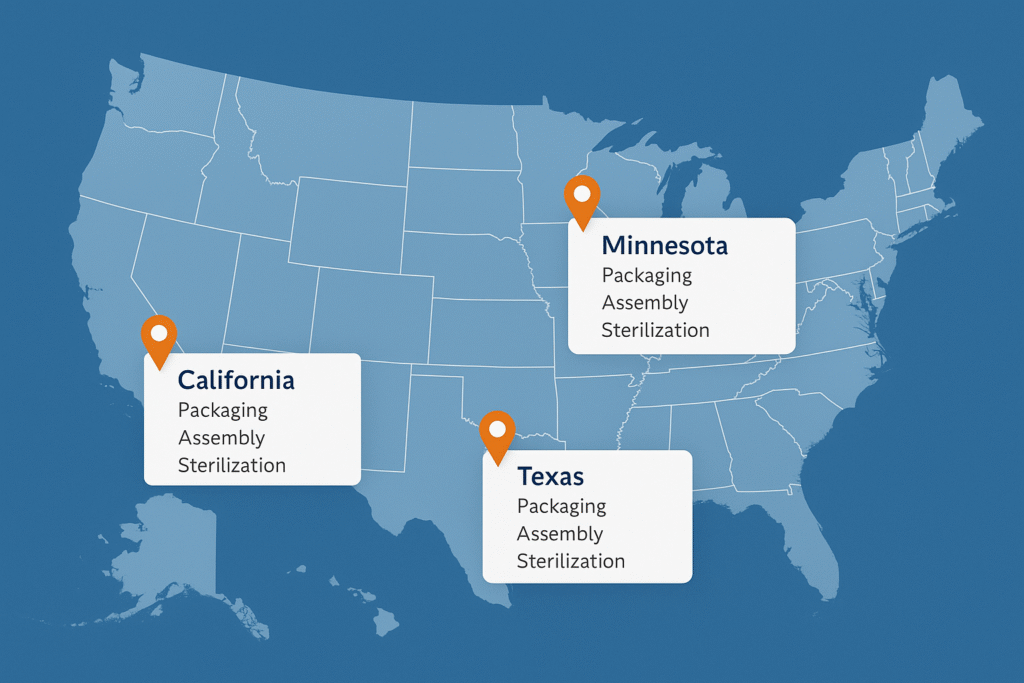
With facilities in California, Texas, and Minnesota, PRO-TECH delivers U.S.-based packaging, assembly, and sterilization capabilities across multiple geographies, giving clients the flexibility to adapt without compromising quality.
Redundancy Is the New Competitive Advantage
One of the most effective ways to mitigate disruption is to build redundancy into your packaging and assembly strategy.
Instead of relying on a single-source supplier or single-site operation, look for partners that offer:
- Multiple facilities with mirrored capabilities
- Standardized SOPs and validations across sites
- Proactive risk assessments and scenario planning
At PRO-TECH, our clients benefit from our multi-site approach, which means that if one facility is affected by local conditions (natural disasters, labor shortages, etc.), production can continue seamlessly at another.
The Role of a Strategic Packaging Partner
Your packaging partner shouldn’t be just a vendor executing orders; they should be an extension of your strategy team.
Key qualifications to look for:
- FDA-registered and ISO 13485 certified
- Multiple domestic locations
- In-house lab and validation services
- Experience with both pouch and tray systems, sterile barrier packaging, sewn products, and assembly
With in-house cleanrooms, environmental stress testing, and decades-long supplier relationships, PRO-TECH helps ensure packaging solutions are not only compliant but engineered for long-term durability in a rapidly shifting market.
“After 45 years in business, we’ve seen just about everything—from economic swings and supply chain bottlenecks to evolving FDA guidelines. What gives our clients peace of mind is knowing that we’ve already solved these challenges—and we’ve built a supply chain model ready for the next ones.”
— Aaron Swanson, President, PRO-TECH Design & Manufacturing, Inc.
The Bottom Line: Resilience Isn’t a Buzzword—It’s a Strategy
Disruptions will happen. The question is whether your supply chain will bend or break. With the right partners, your operations can remain stable, compliant, and responsive, no matter what comes next.
At PRO-TECH, we’re more than a vendor. We’re a strategic partner with the infrastructure, expertise, and adaptability to keep you ahead of the curve.
Let’s Talk Supply Chain Strategy
Is your current packaging partner prepared for what’s next?
Contact us to schedule a consultation.
FAQs: Building a Resilient Medical Device Supply Chain in 2025
- Why are medical device supply chains under more pressure in 2025?
Medical device manufacturers are navigating a perfect storm of challenges—volatile tariffs between the U.S. and major overseas hubs, reshoring and nearshoring trends, geopolitical instability, and heightened FDA, ISO 13485, and UDI compliance demands. This means sourcing decisions now require balancing cost, speed, regulatory alignment, and risk management.
- How does redundancy improve supply chain resilience?
Redundancy means having multiple facilities with mirrored capabilities so production can continue if one site is affected by disruptions like natural disasters or labor shortages. Standardized SOPs and proactive risk planning ensure smooth transitions between facilities without compromising quality or timelines.
- What should I look for in a strategic medical device packaging partner?
The ideal partner should be FDA-registered, ISO 13485 certified, operate multiple domestic locations, offer in-house lab and validation services, and have proven expertise in sterile barrier packaging, tray and pouch systems, sewn products, and assembly. This combination ensures compliance, flexibility, and scalability.
- How can a packaging partner help with regulatory compliance?
A qualified packaging partner aligns operations with FDA and ISO 13485 standards across all facilities, maintains full traceability, and conducts rigorous environmental and stress testing to validate packaging performance. This safeguards both product integrity and market readiness.
- What contingency plans should a manufacturer expect from its supply chain partner?
Plans should include alternative sourcing, multi-site production capabilities, documented risk assessments, and rapid transfer protocols to maintain continuity during regional disruptions, material shortages, or unexpected regulatory changes.
- Why is resilience more than just a buzzword in medical device manufacturing?
Resilience is an operational strategy that allows manufacturers to adapt quickly without compromising quality or compliance. In an era of unpredictable global events, it’s the difference between reacting to disruptions and preventing them from affecting product delivery.

