
by PRO-TECH Design | Apr 22, 2024 | Articles
In recent years, the medical device industry, known for its high standards and constant innovation, has increasingly turned to outsourced manufacturing to satisfy the global need for medical technologies. According to a report by Grand View Research, the global...
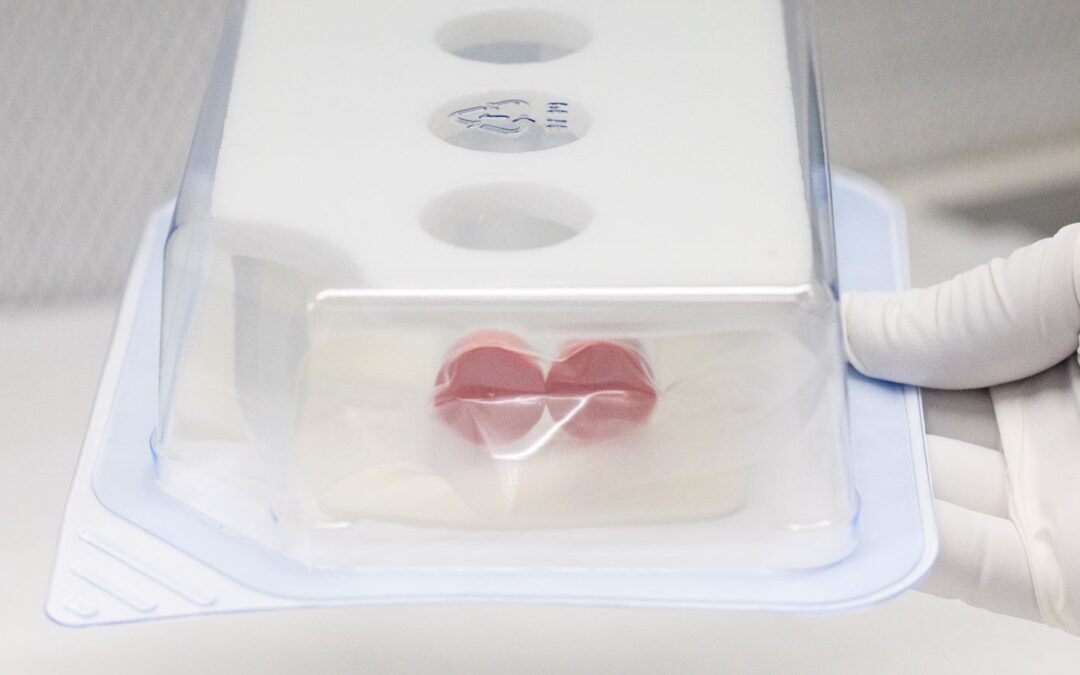
by PRO-TECH Design | Apr 10, 2024 | Articles
The art and science of packaging in the medical device sector are foundational to the industry’s mission of delivering health solutions safely and effectively. This critical aspect of medical device production is not merely about encasing a product; it’s...

by PRO-TECH Design | Mar 6, 2024 | Articles
In today’s world of manufacturing, the decision to partner with a Contract Manufacturing Organization (CMO) can be a game-changer for your business. This choice is not just a matter of outsourcing production; it’s about finding a strategic partner that aligns...

by PRO-TECH Design | Feb 29, 2024 | Articles
Do you know that ASTM standards play a critical role in ensuring compliance for medical device contract packaging? ASTM International, a standards development organization with a global reach, has set the bar for quality, safety, and performance in sterile packaging....

by PRO-TECH Design | Dec 14, 2023 | Articles
Did you know that autoclaves, first invented in 1879, have become an indispensable tool in modern medicine, and one of the most used methods for sterilization in hospitals around the world? In the field of medical device packaging in particular, understanding this...

by PRO-TECH Design | Dec 6, 2023 | Articles
According to the International Irradiation Association, over 40% of all single-use medical devices produced globally are sterilized using gamma radiation. This sterilization method ensures the highest standards of patient safety and the optimal functionality of...







